Novartis, the company that manufactures Excedrin voluntarily recalled the product in December because bottles made at their Lincoln, Nebraska plant may have been contaminated with powerful prescription drugs made at the same factory.
Immediately after the recall, bottles began selling online at sites such as Ebay for as much as $100 a bottle. Since Novartis has still not restocked product, the scarcity of the remaining bottles has skyrocketed to over $300 on the secondary market for bottles containing 100 pills. The retail price of Excedrin for 100 count bottles was $6 before the shortage.
Excedrin, Gas-X and NoDoz and Bufferin products were all recalled and may have accidentally been packaged with prescription painkillers made at the same facility. These opioid drugs include Percocet, Endocet, Opana and Zydone.
There does not seem to be a secondary market for Gas-X, NoDox and Bufferin.
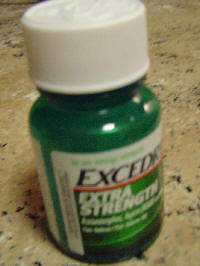
$300 a Bottle
Novartis said mixing of different products in the same bottle could result in taking an incorrect drug or receiving a higher or lower strength than intended or receiving an unintended ingredient, which could result in a reaction or overdose.
The recall is for bottled packages of Excedrin and NoDoz with expiration dates of Dec. 20, 2014, or earlier, and for Gas-X and Bufferin with expiration dates of Dec. 20, 2013, or earlier, sold in the United States.
The products are being recalled due to an internal product review and complaints that identified issues such as broken gelcaps, chipped tablets and inconsistent bottle packaging line clearance practices, which created a potential for a tablet mix up.
The company said it has notified its distributors and retailers. Consumers can find more information on the website www.novartis-otc.com
Novartis has advised that Excedrin, Gas-X and NoDoz and Bufferin products may not return to shelves until July.
Consumers are cautioned to not buy recalled product.










Recent Comments